Marine Threat: Extinction
BACKGROUND
The threat of extinction looms over endangered species. But losing species (defaunation) is not the only problem contemporary ecosystems must contend with; historic extinction events continue to impair ecosystems to this day. Extinctions not only degrade an ecosystem’s biodiversity, but can also degrade complex ecological interactions such as food chains, ecosystem engineering, and mutualistic relationships – leading to reductions in bioabundance and bioproductivity. The impacts that extinction may have on marine ecosystems have been characterized by several seminal experiments in marine ecosystems.
In the late 1960s, Robert Paine experimentally removed different species from several marine sites in the Pacific Northwest of the United States. Many of the “local extinctions” he experimentally created had little impact on biodiversity; however, when the ochre sea star (Pisaster ochraceus) was removed, the mussels on which it primarily preyed overpopulated and pushed out all the other species also inhabiting the rocky surfaces of the tidal zone (Paine 1966), resulting in a far less diverse system that was predominated by a single species. Paine labeled the sea star a “keystone species,” a term he coined to describe the species’ pivotal role in supporting biodiversity. Similarly, in its original architectural usage, a keystone refers the central stone at the top of an archway that locks the arch together, without which the arch topples to the ground.
Marine defaunation has occurred at a much slower rate than that for terrestrial fauna, but it appears that rate is rapidly increasing. The International Union for the Conservation of Nature records 15 extinctions of marine species documented over the past 500 years, including the Steller’s sea cow, Caribbean monk seal, and a single species of fish, the New Zealand grayling. The growing footprint of human society is increasingly impacting the oceans, a fact reflected in the acceleration of marine defaunation. Human activity can be cited as the cause of a number marine extinctions to date; the westward expansion of Europeans led to the extinction of the great auk and the Labrador duck in the mid-19th century. Sadly, it appears the Vaquita will be the next species to join this list, with only 22 individuals left.
Although only a few extinctions have been recorded to date, defaunation of oceans likely has had many widespread impacts. It is likely that temporary historic shifts in habitat around the world, similar to that which contributed to the extinction of the Steller’s sea cow, have led to many extinctions of species that were never recorded. The full extent to which human caused extinctions have altered marine ecosystems may never be fully understood.
An important example of the negative effects of marine defaunation comes from the indirect impacts that historic whaling incurred upon kelp forests decades later (Estes 2004). The decimation of large whales in the 1800s forced orcas to prey on smaller marine mammals, wiping out Steller’s sea lions, then harbor seals, then fur seals, until the only suitable prey remaining were sea otters. This led to a crash in Alaskan sea otter populations in the 1990s, just a decade after the otters had fully recovered from the 19th century fur trade. Without sea otters to keep sea urchin populations in check, the urchin populations boomed and overgrazed kelp forests – creating kelp barrens, which effectively eliminated entire habitats for hundreds of species.
At least one of the fifteen recorded extinctions was caused, in part, by a similar trophic downgrading, the extinction of the Steller’s sea cow (Estes 2016). In addition to the direct pressure from human hunting, the decimation of sea otters during the fur trade set in motion the destruction of kelp forests upon which the sea cows grazed. It is significant that the sea cow’s extinction happened less than three decades after the species was discovered by Europeans in 1741, emphasizing the importance kelp forest loss had on the species. While sea otters eventually rebounded and kelp forests recovered by the 20th century, the sea cows were gone. Scientists can now only speculate how the loss of the sea cow has impacted these recovered kelp forests.
PROGRESS TO DATE
Restoring the roles of extinct species through reintroduction or translocation can have valuable benefits, and can reverse the ecological damage caused by an extinction. Conservationists have reintroduced individuals from a neighboring population; the reintroductions of wolves to Yellowstone National Park is an excellent example. Other times, a related species can replace an extinct species, such as the introduction of the Aldabra giant tortoise on Mauritius to replace the extinct endemic giant tortoise. In each case, unexpected widespread ecological benefits were observed as a result of the restoration.
Restoration of the ecological function of completely extinct species has been impossible. However, thanks to paleogenomics and gene-editing, the practice of “de-extinction” via precise-hybridization may recreate ecotypes of such species. With de-extinction a serious solution to restoring lost biodiversity, discussion of the ethical and ecological implications have prompted the creation of de-extinction criteria. Each candidate for de-extinction should have a functional role in the ecology of an existing ecosystem; the cause of extinction must be removed; and, habitat must be available at a scale for the species to recover.
In the marine environment, two extinct species have been discussed as potential candidates for de-extinction: the Steller’s sea cow, which went extinct in 1768, and the great auk, the only flightless seabird of the North Atlantic Ocean, which went extinct in the 1850s. Both species met the requirements listed above. While still poorly understood because of how long each species has been extinct, the specific food chain and ecosystem implications are expected to be significant. The severe hunting pressure the led to each species’ extinction is no longer a threat, and extensive habitats are available for the recolonization of de-extinct Steller’s sea cows and great auks.
Sufficient DNA from the Steller’s sea cow has been successfully sequenced to reveal that the dugong is the closest living relative. The DNA is of sufficient quality that an entire genome sequence is likely attainable. Therefore, in the first step of de-extinction research, comparative genomics will be used to discover the genes responsible for cold climate adaptation and gigantism.
For any genetic rescue project involving gene editing, the major bottleneck is implementing reproductive techniques to progress from in vitro to in vivo. For placental mammals, like the Steller’s sea cow, somatic cell nuclear transfer (aka cloning), offers a proven method ( increasingly common for livestock) for creating embryos from gene-edited cells. But to date, no attempts have been made to clone a marine mammal. Other reproductive technologies for mammals in development include stem cell embryogenesis.
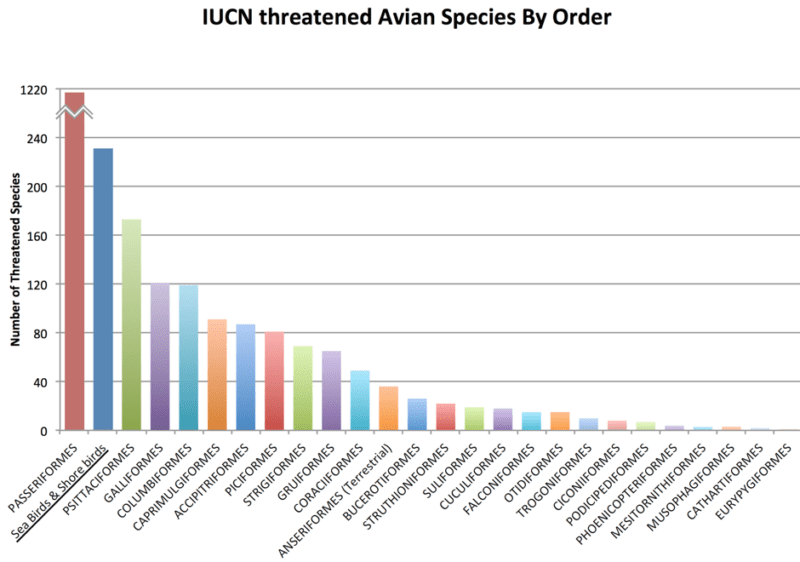
While restoring the great auk ecotype to the North Atlantic Ocean would potentially initiate a cascade of beneficial trophic and habitat changes in that ecosystem, the most important benefit of de-extinction would be the development of biotechnologies that can be used for the genetic rescue of some endangered species of seabirds and shorebirds, which are among the most endangered groups of living birds (Pictured Below).
According to IUCN, seabirds and shorebirds comprise one of the largest groups of conservation concern among avian diversity. Seabirds and shorebirds comprise the orders Charadriiformes (100 species), Procellariiformes (83 species), Pelecaniformes (27 species), Sphenisciformes (13 species), Gaviiformes (1 species), and Anseriformes (7 species).
INNOVATION
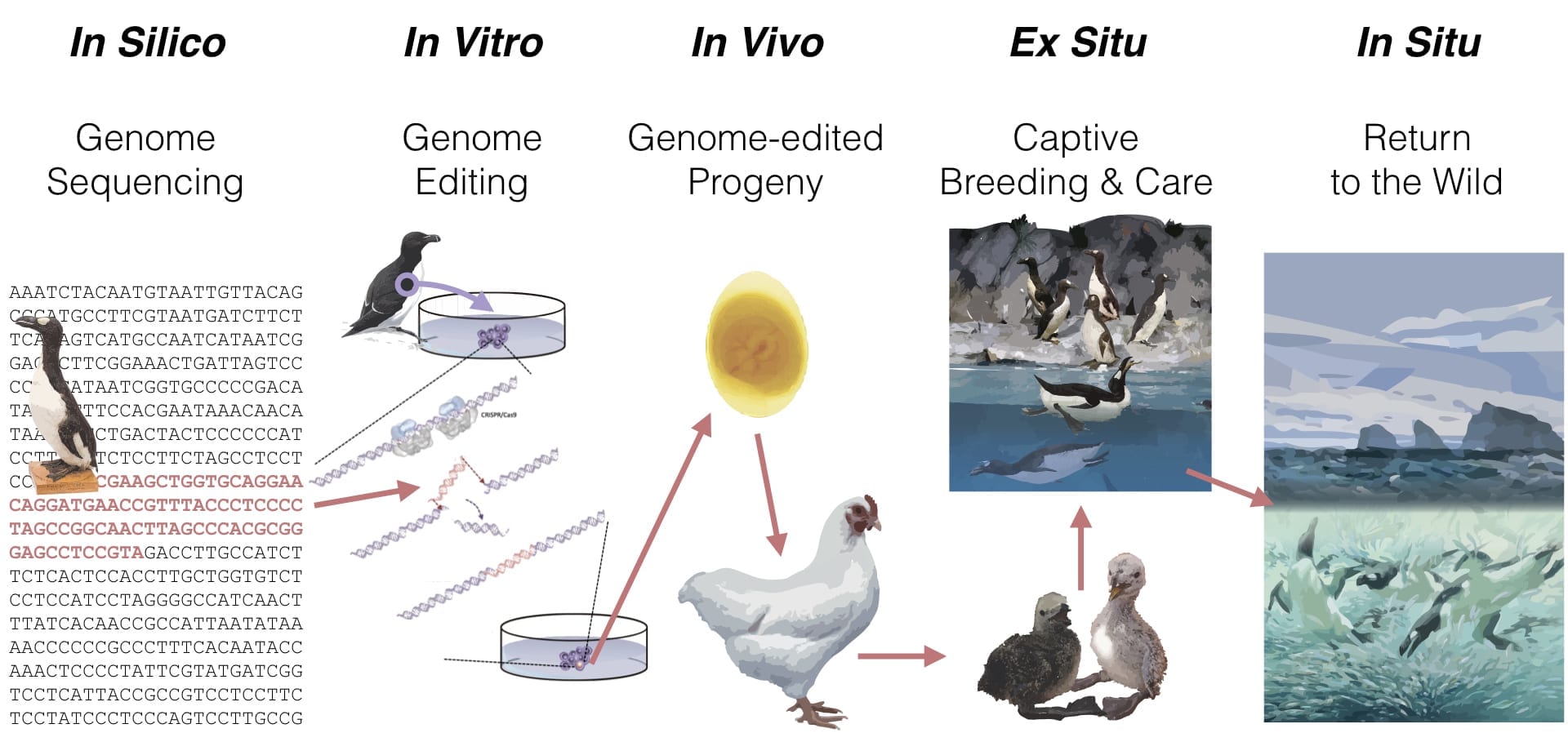
The de-extinction process (graphically shown for the great auk below) for any species has five phases:
-
- In Silico: Genomic sequencing of the extinct species and the living template species.
- In Vitro: Reproductively competent cells of the template species edited to possess the genetic traits of the extinct species.
- In Vivo: Advanced reproductive technologies used to generate gametes and/or embryos from the gene-edited cells.
- Ex situ: De-extinct species propagated in captivity for release to the wild.
- In Situ: De-extinct species released to the wild with managed and monitored recovery.
Great Auk De-Extinction
Developing Interspecies Germ-line Transmission for Seabirds
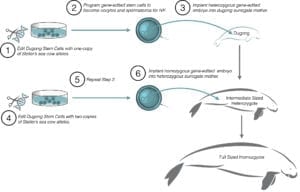
The only viable reproductive technology in birds useful to genetic rescue is the germ-line transfer/transmission of cultured primordial germ cells (PGCs), outlined in Figure 6. This technology has only been developed for the domestic chicken, and limited attempts at using these techniques with other species have yet to be successful, creating a technological barrier for the application of genetic technologies to avian conservation.
Germ-line Transmission works as follows:
- PGCs are isolated from a donor embryo and then cultured in vitro.
(Cultured PGCs can also be cryopreserved for biobanking or gene-edited for genetic intervention.) - Donor PGCs are then injected (transferred) into a developing recipient embryo, which can be the same or a different species. The example in Figure 2 uses a chicken. This creates what is known as a “germ-line chimera.” The germ-line chimera has the physical appearance of the recipient species, but carries the PGCs of the donor species in its reproductive system.
- The germ-line chimeras are bred to produce offspring of the donor lineage.
Steller's Sea Cow De-Extinction
Developing Advanced Reproductive Technologies for Marine Mammals
For placental mammals like the Steller’s sea cow, cloning is a universal method for creating embryos from gene-edited cells. But to date, no attempts have been made to clone a marine mammal. Stem cell embryogenesis, in which stem cells are programmed to develop into oocytes and spermatozoa for in vitro fertilization (IVF), is also a promising pathway because embryos generated by IVF have higher rates of successful implementation and development. There is also no need to collect oocytes for generating embryos, a challenge for cloning a species where such reproductive resources are limited. While stem cell embryogenesis solves that problem, the technique is unperfected for any species other than mice, although scientists at San Diego Zoo Global are developing techniques for white rhinoceros.
Regardless of which technique is used – cloning or stem cell embryogenesis – one of the first major technical hurdles for Steller’s sea cow de-extinction is the successful implantation of an embryo into a dugong surrogate mother.
Read MoreRISKS & CHALLENGES
Developing PGC culture and germ-line transmission techniques for birds is “high risk/high reward” tech development. The initial stages are resource-intensive, and there are still large knowledge gaps in PGC cellular biology and reproductive physiology of most avian species. To date, techniques that have worked in domestic chickens have failed with other birds, possibly due to the chicken’s long history of selective breeding.
Exacerbating the complexities of PGC cellular biology and reproductive physiology, the “low tech” components of animal reproductive technologies – the animal husbandry and egg handling – can be even more challenging. For wild birds, animal husbandry, semen collection, artificial incubation, and embryological developmental stages are typically poorly characterized or lacking any foundational knowledge. These efforts will require careful selection of model species to build the foundational animal science knowledge necessary to enable PGC culture. It is for this reason that the button quail was highlighted as a possible model organism for the development of interspecies germ-line transmission techniques.
LEADERS
Leaders in avian genetic engineering and reproductive technologies are:
- Academic laboratories led by Michael McGrew and Helen Sang at the Roslin Institute
- A governmental laboratory group led by Tim Doran at the Commonwealth Scientific and
- Industry Research Organization’s Australian Animal Health Laboratory (CSIRO AAHL).
- Revive & Restore’s avian de-extinction program, led by Ben Novak who is currently working with the CSIRO AAHL to advance gene-editing research in pigeons.
- The Schusser lab at Technische Universität München, which is attempting to transmit cultured spermatogonial cells of the greater prairie chicken through domestic chicken interspecies germ-line chimeras.
- Texas A&M University (TAMU), a leader in exotic bird research, is developing a programmatic focal area on PGC culture and transmission for diverse wild bird species.
REFERENCES
Estes, James A., Burdin, A., Doak, D.F. 2016. Sea Otters, Kelp Forests, and the Extinction of the Steller’s Sea Cow. PNAS 113(4): 880-885
Estes, James A., et al. “Trophic downgrading of planet Earth.” Science 333.6040 (2011): 301-306.
Estes, James A., et al. 2004. Complex Trophic Interactions in Kelp Forest Ecosystems.Bulletin of Marine Science 74(3): 621-638.
Fritts, Steven H., et al. “Planning and implementing a reintroduction of wolves to Yellowstone National Park and central Idaho.” Restoration Ecology 5.1 (1997): 7-27.
Hansen, Dennis M., et al. “Ecological history and latent conservation potential: large and giant tortoises as a model for taxon substitutions.” Ecography 33.2 (2010): 272-284.
McCauley, Douglas J., et al. “Marine defaunation: animal loss in the global ocean.” Science 347.6219 (2015): 1255641.
Moum, Truls, Ulfur Arnason, and Einar Arnason. “Mitochondrial DNA sequence evolution and phylogeny of the Atlantic Alcidae, including the extinct Great Auk (Pinguinus impennis).” Molecular Biology and Evolution 19.9 (2002): 1434-1439.
Novak, Ben. “De-Extinction.” Genes 9.11 (2018): 548.
Olson, Storrs L., and Judith N. Lund. “Whalers and woggins: a new vocabulary for interpreting some early accounts of the great auk and penguins.” Archives of natural history 34.1 (2007): 69-78.
Olson, Storrs L., and Judith N. Lund. “Additional references to “woggin” as a name for penguins.” Archives of natural history43.2 (2016): 360-362.
Paine, Robert T. 1966. Food Web Complexity and Species Diversity. American Naturalist 100(910): 65-75.
Paine, Robert T. 1969. A Note on Trophic Coplexity and Community Stability. American Naturalist 103(929): 91-93.
Payne, Jonathan L., et al. “Ecological selectivity of the emerging mass extinction in the oceans.” Science 353.6305 (2016): 1284-1286.
Ripple, William J., and Robert L. Beschta. “Trophic cascades in Yellowstone: the first 15 years after wolf reintroduction.” Biological Conservation 145.1 (2012): 205-213.
Springer, Mark S., et al. “Interordinal gene capture, the phylogenetic position of Steller’s sea cow based on molecular and morphological data, and the macroevolutionary history of Sirenia.” Molecular Phylogenetics and Evolution 91 (2015): 178-193.
Thomas, Jessica E., et al. “An‛ Aukward’Tale: A Genetic Approach to Discover the Whereabouts of the Last Great Auks.” Genes 8.6 (2017): 164.
Van de Lavoir, Marie-Cecile, et al. “Germline transmission of genetically modified primordial germ cells.” Nature 441.7094 (2006): 766.
Van de Lavoir, Marie-Cecile, et al. “Interspecific germline transmission of cultured primordial germ cells.” PLoS One 7.5 (2012): e35664.
Vermeij, Geerat J. “Biogeography of recently extinct marine species: implications for conservation.” Conservation Biology7.2 (1993): 391-397.
Wernery, Ulrich, et al. “Primordial germ cell-mediated chimera technology produces viable pure-line Houbara bustard offspring: potential for repopulating an endangered species.” PLoS One 5.12 (2010): e15824.





Back to Top