Who Decides
Other sections of this Horizon Scan focus on what could be possible if genomic technologies were applied to the myriad of challenges facing marine conservation. Here the focus is on “who decides” if, when, and how these technologies might be developed and used.
INTRODUCTION
The spectrum of biotechnologies that could be developed for conservation applications present a diverse spectrum of ethical, social, and legal challenges and opportunities. For instance, genomic insight for the management of fisheries is a precise and relatively uncontroversial tool to understand the population dynamics of a fishery. In contrast, genome engineering that limits the reproductive potential of an invasive species is controversial because of the intervention in a natural evolutionary process and may have far-reaching and unintended consequences. No matter the level of intervention, responsible innovation will require that potential benefits and risks be weighed with objectivity alongside thoughtful consideration of ethical concerns.
It is an essential question to ask: when should humankind intervene in a natural evolutionary process. Oceans are shared environments, and technological interventions should be developed in a manner that reflects the values and needs of the communities that depend upon and are in relationship with shared marine ecosystems.
Biotechnology developed for genetic interventions should be applied only with widespread multi-stakeholder engagement. This outreach process should take place early enough to allow the project design to be modified with the benefit of feedback from regulators, insurers, industry, and civil society. Questions pertaining to the purposes, methods, and potential implications of any potential intervention should be asked at the very earliest stages of research, with the intention to integrate the environmental, economic, health, safety, security, ethical, and governance aspects of biotechnologies into the design of the project.
Genetic engineering solutions for wildlife are both scientifically and ethically different in many ways from agricultural and medical applications, making it difficult to transfer conversations from the USDA and FDA to wildlife applications. Fundamentally, nature and natural systems are held in the public trust as a shared asset. This represents a wholly different ethical context from which to consider the ramifications of genetic interventions. Being cognizant of these long–standing regulatory frameworks is necessary as new paradigms are evolving in parallel with potential genomic applications in conservation. Therefore, there is an opportunity to be deliberate and systematic in how we develop these systems to serve both the public and ecosystems.
Ideally, any genomic intervention in marine settings will be shaped by a multi-tiered international regulatory system that attempts to systematically balance the potential benefits of innovation against associated risks. Therefore, early engagement of diverse disciplines (ethicists, scientists, conservationists, regulators and policy makers) and stakeholders across civil society must play a role in evaluating genomics as a solution set for ocean conservation. With objective and inclusive engagement and stringent risk assessment, it should be possible to develop safe and appropriate genetic solutions that turn the tide on our damaged oceans.
CHALLENGES OF GENETIC INTERVENTIONS FOR WILDLIFE CONSERVATION
Genetic engineering solutions for wildlife are both scientifically and ethically different in many ways from agricultural and medical applications, making it difficult to transfer conversations from the USDA and FDA to wildlife applications. Current regulatory policies aim to minimize the risk of environmental harm from the release or human consumption of a GMO. In contrast, for conservation efforts, the goal will be to have a large persistent beneficial impact on the environment, while also minimizing any negative impacts. This should be obvious, but the difference in desired outcome will necessarily require changes to the regulatory approval framework, as well as new methods for assessing long-term environmental impacts.
The specter of “unintended consequences” is a valid reaction to the abundant evidence of non-native invasive species that when released (intentionally or unintentionally) took over whole ecosystems, disrupted food chains and other ecological functions, or completely displaced critically important native species. However, modern conservation practice has developed norms and practices of planned biocontrol, re-introductions and translocations. It is necessary to distinguish between well-planned and intentional releases and those of the past that were poorly thought out. Also, genetic interventions may require additional inquiry into the ecological fitness of the modified organism in a specific ecological setting.
The release of any organism into the environment, whether to suppress, restore, or transform a wild species population, will likely create ripple effects through an ecosystem. For example, use of a genetically engineered mosquito to suppress its counterparts in the wild population could have negative impacts on the bird and fish species that depend on either mosquito adult or larvae, respectively, as a food source. Ecological risk assessments (ERAs) can be used to estimate the probability that an introduced organism would have adverse effects on non-targeted components (this includes other species) in a specific environment. To be useful for conservation applications, they will need to estimate positive environmental impacts, as well. The methods used to construct ERAs will also need to be updated so they can accurately predict ecological effects over increased spatial and temporal scales. The expertise of ecologists will be integral to successful genetic rescue projects; impacted food networks and the relational nature of species-species interactions and dependencies within those networks must be fully resolved to ensure a genetic technology fulfills its function to improve ecosystem health.
Another consideration unique to environmental applications of GMOs is the assessment of alternative approaches. Current policy compares new GMO technologies to existing equivalents to determine if they will be substantially equivalent or better. These considerations are usually part of the “alternatives analysis” required during a permitting process. In conservation applications for wild release, the evaluation of viable alternative methods should occur before the pursuit of a genetic intervention is even considered. In particular, more conventional conservation strategies should be thoroughly evaluated prior to the pursuit of genetic interventions.
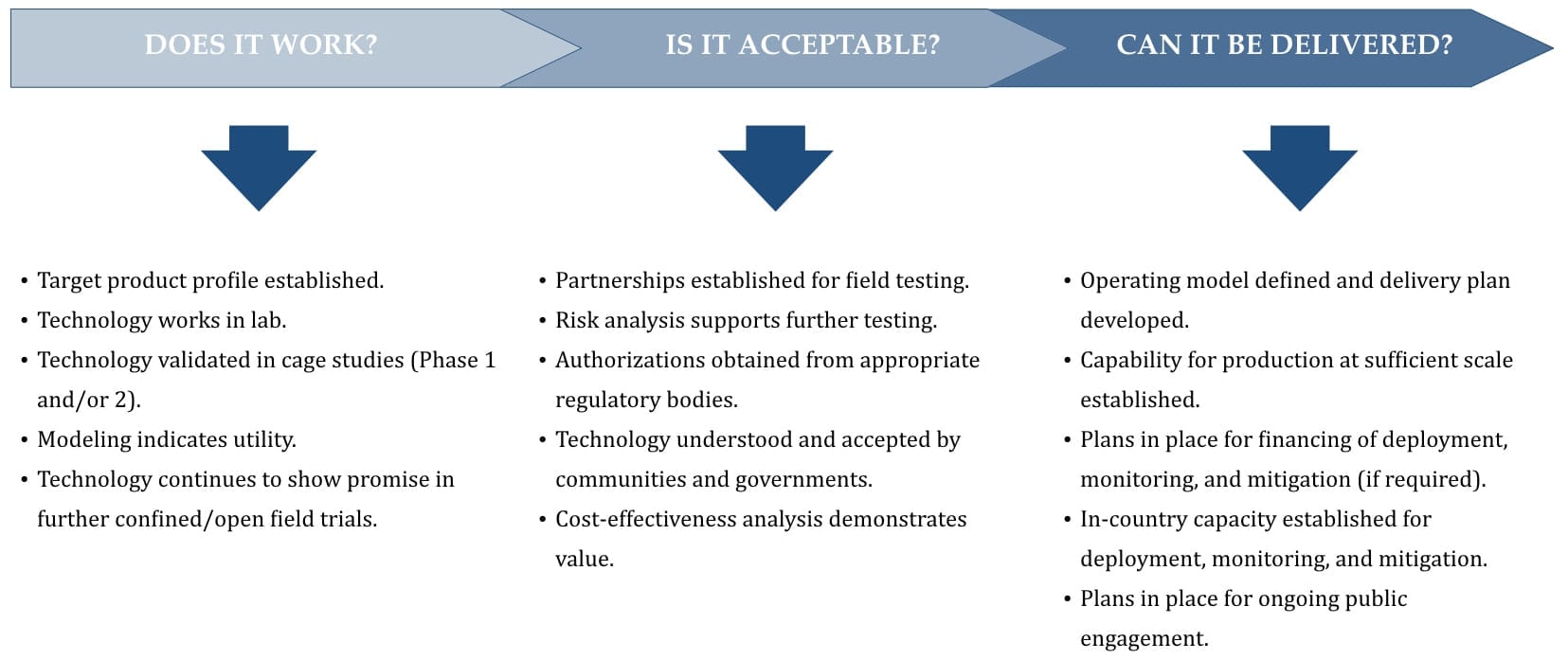
FIGURE 1 — The elements of the critical path for developing and deploying genetically modified organisms (World Health Organization 2014).
A number of U.S. government agencies and conservation NGOs should be involved in the drafting of our national policy and regulation, including the USFWS, EPA, and the Council on Environmental Quality. These agencies currently lack a clear framework for considering new genetic rescue tools. Overly specific regulations may undermine the customization necessary for implementing genetic solutions for diverse conservation applications, while overly vague policies will leave projects in limbo. A systematic framework for evaluating a wide variety of new genetic technologies for environmental applications will be needed to measure the safety and efficacy of these potentially impactful technologies (see Figure 1).
Planners should carefully consider which stakeholders to include and how they could influence the progress of a project.
Broad Stakeholder Engagement: An opportunity to integrate values into project design from the beginning.
Building public trust and engaging feedback will be essential elements of conservation efforts involving genomic technologies. These processes will depend upon relationship-building and should be built on procedures that earn the respect and trust of the public. Authentically incorporating stakeholders into the decision-making process requires the design of mechanisms for input, which in turn may necessitate transparency in decision-making. Responsible development of genetic rescue strategies will therefore depend upon an exploration of the underlying values that are motivating the project and shaping its design. For example, how decision-makers relate to their natural environment will form the ethical justification for many of these interventions. Whether the ecosystem under consideration is perceived to have utilitarian value (i.e. valued for its benefits to humans) versus intrinsic value (value is independent of human benefit) could impact the resources and care available to save that ecosystem (Batavia and Nelson 2017).<
Similarly, the value that decision-makers place on technology will have an enormous impact on outcomes. Some will see technology as something separate from nature and thus want to limit human technological intervention in “natural” processes. Others will be more open to using genetic strategies to conserve and restore nature. Therefore, it will also be important to explore alternative approaches and their respective ethical responses. Comparing and contrasting alternative approaches may allow decision-makers and the public to understand that deciding not to use a genetic technology may have equally, if not larger, negative consequences for marine ecosystems. During multi-stakeholder engagement activities, it will be important to openly explore these values and provide neutral space that allows for listening and reflection. Engaging diverse perspectives and value-systems may help create a path forward that is both fair and effective.
GMO foods and labeling provide a cautionary example of why transparent open dialogue with the public is so important. Despite the near universal acceptance of GMO foods as safe by the scientific community, most people in the general public consider them to be less healthy, or even dangerous to consume than “organic” or “natural” foods. Common GMO misconceptions are that they contain chemicals, that they are made with pesticides, or that they will cause allergic reactions or even mutations. Many scientists believe that a better-informed public and more genetic education would alleviate public pushback. But in fact, new research on cultural cognition suggests that distrust of technology or even scientific consensus on issues like climate change can correlate with high degrees of scientific literacy (Kahan 2014). Other ethical and social dimensions beyond safety may also inform public opinion, including anti-corporate sentiment and social justice issues.
Thus, it will be important to inform and engage the public, not only so the technology is clearly understood, but also so that the public may have to opportunity to convey the value systems that shape their perceptions of a proposed technology. Each of the marine conservation issues described in the following sections provides a potential opportunity to plan, develop and test mechanisms for coupling the science and technology with ethical and social perspectives and regulatory frameworks.
Suggested Engagement Principles for Considering Genomic intervention for Conservation
- Weighing Risks and Benefits: Decision makers should conduct a thorough review of both the intended outcomes and the potential benefits of a genomic intervention and the potential harm and downstream risks. Questions to consider include: Have other, more established interventions been tried and failed? Do other interventions, such as the application of antibiotics or pesticides, have potentially worse environmental harms? Is it the most efficacious, lasting, and least risky solution to an environmental problem, not merely an equivalent or novel approach?
- Transparency: Decision makers should proactively identify and inform key stakeholders in the early stages of technology development. This includes partners from the private sector (particularly biotech firms), social science experts, public sector, international organizations, research organizations, religious and ethical organizations, NGOs, and local communities. This should involve objective discussions with the public about the risks and potential impacts of proposed environmental solutions and also subjective discussions about values.
- Procedures: Decision makers should conduct systematic and data-driven reviews of recommended best practices. These should include surveys of recent intentional environmental releases of organisms (biocontrol, re-introductions and translocations) into natural environments and studies of the resulting long-term effects.
- Tests: Decision makers should first field-test genomic technologies in contained environments to minimize unintended environmental harm. These should be simple enough to be cost-effective, yet complex enough to sufficiently mimic natural ecosystems to yield useful data on the efficacy of developing technologies. Suitable test environments will be especially important for marine environments.
- Predictions: Decision makers should employ computational models, particularly as they grow more sophisticated with time, to predict the long-term effects on ecosystems and highlight potential failures of specific applications of genetic technologies.
- Measures: Decision makers should employ standard metrics for measuring safety and efficacy at appropriate environmental scales, so that direct comparisons of data sets can be made and the universal analytic tools be developed.
- Protocols: Decision makers should understand the regulatory approval processes and jurisdictions that currently guide the transition of new technologies from the lab to field trials, and should be prepared to help guide new policies when needed.
- Public-Private Partnerships: Decision makers should collaborate with philanthropists, NGOs, agencies, and community groups in the implementation of these technologies to evaluate risks, to address the controversies, and to gain critical stakeholder support. These sectors can convene conversations, commission studies, identify priorities, and connect the emerging technology with civil society to identify safeguards.
- Remediation: Decision makers should prepare for the possibility of failure. In the event of unintended consequences, will it be possible to regain control of the organism? Studied possibilities for this include the creation of gene drives with “off switches.”
- Learnings: Decision makers should continuously monitor the introduced organism and its environment once the technology has been deployed. Because these technologies remain new, decision makers should proactively share their findings and invite researchers to learn from their successes or failures. Ultimately, this transparency will allow successful intervention techniques to be more rapidly adopted globally.
Regulation of Biotechnology
Background: The invention of recombinant DNA technologies in the 1970s led the United States (U.S) and other countries to take an active role in regulating the research, development, and release of genetically-modified biological products (Berg et al. 1975). Determining a biotechnology’s risk and whether it’s safe for the public and the environment currently is primarily the task of federal regulatory agencies. The U.S., Australia, and the European Union (E.U.), all have regulatory frameworks largely centered on methods of containment, that address genetically modified organisms. Therefore, applications that aim for the intentional release of genetically altered organisms into uncontained, wild ecosystems place strain on existing frameworks.
In the U.S., no single agency is responsible for overseeing genetically engineered organisms. The Coordinated Framework for Regulation of Biotechnology governs regulatory policy; approval falls to either the Food and Drug Administration (FDA), the Environmental Protection Agency (EPA), or the Department of Agricultural (USDA), depending on the intended function of the GMO (U.S. Office of Science and Technology Policy 1986). In contrast, Australia has attempted to centralize GMO oversight, in part through the establishment of its Office of the Gene Technology Regulator, which alongside appropriate government agencies and under the Gene Technology Act 2000 would oversee contained field trial studies, as well as eventual intended release of any genetically modified organism (Australian Academy of Science 2017). Regulation procedures and outcomes of novel biotechnologies intended for marine conservation are likely to vary significantly between nations. We discuss the international regulatory climate later on in this chapter.
Challenge / Opportunity: Current U.S. policy focuses on the products under review, rather than on the methods that were used to create the product. Therefore, regulation of new gene editing techniques and gene drives intended for environmental conservation will fall under existing U.S. biotechnology policy.
However, recent attempts by British technology company Oxitec to gain U.S. regulatory approval for field trial assessment of their proprietary genetically engineered mosquito to suppress wild vectors of dengue fever and Zika underscores a need for better interagency coordination between U.S. regulatory agencies (Meghani and Kuzma 2018), as well as more robust public engagement procedures (Neuhaus and Caplan 2017). With significant omissions in both the draft risk assessment and preliminary “Findings of No Significant Impact,” the FDA should make significant changes to its risk evaluation protocols to allow for a broader study of possible ecological impacts and to survey public constituencies to determine which values should shape the risk assessment of genetically engineered organisms (Meghani and Kuzma 2018). By mandating that the agency must engage the normative concerns of the public, future risk assessments will be less likely than current protocols to advance the biotechnology industry’s interests without the public’s scrutiny and consent. This transparency would create a more democratic risk assessment process that controls for unacknowledged and unchallenged biases, which would result in more rigorous risk assessments. Attempts are currently underway to modernize U.S. agency procedures to address novel genetic technologies.
Traditional genetic engineering and the new techniques of precise gene editing may end up following different regulatory paths. For example, it looks as though the U.S. may not even regulate organisms that have been subjected to gene editing if the resultant genomic changes are indistinguishable from mutations that could have occurred through traditional breeding strategies. In contrast, the E.U. has decided to regulate gene edited organisms, no matter the level of changes made, as genetically modified organisms. Thus, any proposed genetically engineered organism will face an evolving regulatory path depending on the nation, the organism being altered, the alterations made, and its intended function. Such uncertainty makes planned engagement and comprehensive consideration more difficult. Additional foresight is needed to avoid ambiguity and administer relevant regulatory frameworks for conservation applications.
Control and Regulation of Research Involving Genetically Modified Organisms (GMOs)
Most universities and research institutes have special committees that are responsible for approving experiments involving genetic engineering. Some experiments, like contained field trials also need permission from national regulators. The development of genetic technologies specifically involving endangered species could also fall under the jurisdiction of the USFWS and the Endangered Species Act. It remains to be seen if the USFWS will develop policy that can specifically address novel genetic technologies for species conservation, invasive species control and/or restoration of threatened species.
To facilitate the rapid pace of research and innovation, most countries now have administrative exemptions for GMOs that only pose a low risk, including standard laboratory model organisms and organisms with no pathogenic impacts on humans. Work is underway to assess whether existing research regulations are appropriate for emerging conservation biotechnologies. The U.S. National Academies of Science recently reported that current laboratory containment protocols and biosafety procedures for contained field trials are adequate (with some modification) to safeguard gene drive research (National Academies of Sciences, Engineering, and Medicine 2016).
However, containment in marine settings are objectively more challenging since creating impenetrable, fully sealed environs for field trial studies will be difficult to achieve. This “shared” nature of the marine realm raises the bar on innovation to accommodate these biosafety concerns. Risk mitigation will be critical to ensure safe research. For example, gene drive researchers are developing reversible gene drives and genetic “off-switches” that could be employed in the case of unintended release or escape. Remediation protocols—whether for gene drive expressing rodents or genetically engineered microbes for carbon-sequestration—should be an integral component of any project’s design.
International Regulatory Coordination
International regulation of genetically modified organisms varies widely from country to country. The ocean is a shared global resource without clear boundaries, which will make coordinated international regulation critical. For the synergistic global threats that are impacting these shared marine habitats, genetic interventions may provide innovative targeted responses. Therefore, it is worth the time and effort to forge new alliances to consider these tools.
Several international treaties and policies exist for the regulation of migratory wildlife and marine environments that are likely to impact global governance of novel marine biotechnologies:
- The Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), 1973, is an international agreement to ensure that the trade in wild animals and plants does not threaten their survival.
- The objectives of the 1992 Convention on the Conservation of Biological Diversity (CBD) were the conservation of biological diversity, sustainable use, and the fair and equitable sharing of the benefits arising out of the use of genetic resources, particularly those of marine environments. The Cartagena Protocol, discussed in more detail below, is a particularly relevant portion of this agreement.
- The Nagoya Protocol was a supplemental agreement to the CBD that aimed to implement the fair and equitable sharing of benefits of genetic resources. These and other existing relationships can serve as a foundation or model for the international collaborations that will need to be pursued before genetic rescue efforts are attempted in ocean environments.
There have been recent proposals for improved global oversight of genetically engineered organisms designed to spread in the wild (Kofler et al. 2018). The International Union for the Conservation of Nature (IUCN) is in the process of developing policy that can address genetic engineering of wild species, including genetic rescue of marine ecosystems. However, at this point in time, global governance would largely fall to the UN Convention of Biological Diversity (CBD), which under its Cartagena Protocol oversees transboundary transport of living modified organisms. The 198 signatory parties to the CBD are in the process of updating the Cartagena protocol to specifically address genetic strategies for environmental conservation, including gene drives. Given that genetic interventions in marine settings present a specific set of potential risks, there is merit in building dedicated teams to explore these possibilities. Negotiations are likely to take considerable time, on the scale of 2-6 years, due to the polarized debate surrounding gene drive technology. In the meantime, soft governance proposals that call for responsible innovation (Stilgo 2013), self-imposed ethical frameworks for technologists (Emerson et al. 2017), engagement of impacted communities (Najjar et al. 2017), and multi-stakeholder inputs (Jasanoff and Hurlbut 2018) are likely to be the main structures that serve to safeguard genetic conservation technologies at the international level and hopefully ensure benefits are realized.
CONCLUSION
The innovative field of genomic technologies for ocean conservation has huge potential with diverse and innovative strategies that provide targeted responses to difficult and urgent needs. With these technologies still developing, responsible development necessitates engaging diverse viewpoints and expertise to steer the course. Policy developments often lag behind technology innovation. However, it’s not too soon to anticipate high potential applications for genomic technologies in ocean conservation and begin the necessary preliminary discourse now. The goal should be to iteratively craft a set of guidelines that clearly communicate the social and ethical priorities of all affected societies, and articulate the full range of standards that genetic interventions must meet to be considered for environmental release. Such a framework would open the door to many new, powerful, and potentially less intrusive approaches to long-term environmental restoration and health. However, functional and effective guidelines need to be created through an iterative process. Therefore, regulatory and governance frameworks will be developed and tested in concert with the technologies through projects and case studies.
The hopes and concerns of scientists, the public, and regulators must always be taken into consideration. Balanced deliberation that engages multiple stakeholders will help to ensure genomic interventions develop with thoughtful safeguards and monitoring.
References
Australian Academy of Science. Synthetic Genes in Australia: Implications of Emergent Technologies. May 2017. Web. https://www.science.org.au/files/userfiles/support/documents/gene-drives-discussion-paper-june2017.pdf. Accessed 1 Feb 2019.
Batavia, Chelsea, and Michael Paul Nelson. “For Goodness Sake! What Is Intrinsic Value and Why Should We Care?” Biological Conservation, vol. 209, 2017, pp. 366–376., doi:10.1016/j.biocon.2017.03.003.
Berg, Paul, et al. “Summary Statement of the Asilomar Conference on Recombinant DNA Molecules.” Proceedings of the National Academy of Science of the United States of America, vol. 72, no. 6, 1975, pp. 1981-4.
Emerson, Claudia, et al. “Principles for Gene Drive Research.” Science, vol. 358, no. 6367, 2017, pp. 1135–6., doi: 10.1126/science.aap9026.
Endres, Bryan A. “‘GMO:’ Genetically Modified Organism or Gigantic Monetary Obligation? The Liability Schemes for GMO Damage in the United States and European Union.” Loyola of Los Angeles International and Comparative Law Review, vol. 453, no. 22, 2000. Accessed 1 Feb 2019.
Funk, Cary, and Lee Rainie. “Public and Scientists’ Views on Science and Society.” Pew Research Center, 29 Jan 2015, http://www.pewresearch.org/science/2015/01/29/public-and-scientists-views-on-science-and-society/
Infectious Disease News, February 2018. “How Transparent Should Gene Drive Research Be?” Healio, SLACK Incorporated, www.healio.com/infectious-disease/emerging-diseases/news/print/infectious-disease-news/{e5da0f8d-bb23-40aa-8f2d-0baec3284c35}/how-transparent-should-gene-drive-research-be. Accessed 1 Feb 2019.
Jasanoff, Sheila, and J. Benjamin Hurlbut. “A Global Observatory for Gene Editing.” Nature, vol. 555, no. 7697, 2018, pp. 435–7., doi: 10.1038/d41586-018-03270-w.
Kahan, Dan M. “Climate-Science Communication and the Measurement Problem.” Advances in Political Psychology, vol. 36, no. S1, 2015, pp. 1–43., doi: 10.1111/pops.12244.
Kofler, Natalie, et al. “Editing Nature: Local Roots of Global Governance.” Science, vol. 362, no. 6414, 2018, pp. 527–529., doi:10.1126/science.aat4612.
Meghani, Zahra, and Jennifer Kuzma. “Regulating Animals with Gene Drive Systems: Lessons from the Regulatory Assessment of a Genetically Engineered Mosquito.” Journal of Responsible Innovation, vol. 5, sup. 1, 2017, pp. S203–S222., doi:10.1080/23299460.2017.1407912.
Najjar, Devora A., et al. “Driving Towards Ecotechnologies.” Pathogens and Global Health, vol. 111, no. 8, 2017, pp. 448–58., doi: 10.1080/20477724.2018.1452844. Epub 2018 Apr 9. Accessed 1 Feb 2019.
National Academies of Sciences, Engineering, and Medicine. “Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values.” National Academies P, 2016.
Neuhaus, Carolyn P, and Arthur L. Caplan. “Ethical Lessons From a Tale of Two Genetically Modified Insects. Nature Biotechnology, vol. 35, 2017, pp. 713–6., doi: 10.1038/nbt.3927.
Stilgoe, Jack, et al. “Developing a Framework for Responsible Innovation.” Research Policy, vol. 42, no. 9, 2013, pp. 1568–80., doi: 10.1016/j.respol.2013.05.008
United States. Office of Science and Technology Policy. “Coordinated framework for regulation of biotechnology.” Federal Register, vol. 51, no. 123, 1986, pp. 23302-50.
World Health Organization, et al. Guidance Framework Testing of Genetically Modified Mosquitoes. WHO Document Product Services, Geneva, 2014, pp. 1–159, https://www.who.int/tdr/publications/year/2014/guide-fmrk-gm-mosquit/en/. Accessed 1 Feb 2019.




Back to Top