Big Ideas
CONTEXT
Extinctions not only degrade complexity of an ecosystem’s biodiversity, but can also degrade complex ecological interactions such as food chains, ecosystem engineering, and mutualistic relationships. Restoring the roles of extinct species can be extremely valuable to conservation and is an increasingly considered a solution for ecological problems that stem from past extinctions. When populations go extinct locally, conservationists can reintroduce individuals from a neighboring population (e.g. wolves to Yellowstone National Park), or replace an extinct species with a related species (e.g. the Aldabra giant tortoise on Mauritius).
However, until recently, the roles of completely unique extinct species – species that have no living substitutes – have been impossible to restore. Thanks to paleogenomics and gene-editing, the developing practice of “de-extinction” via precise-hybridization may recreate ecotypes of such species. For marine environments, the extinct Great Auk (the only flightless seabird of the North Atlantic Ocean) has emerged as a potential de-extinction candidate.
De-extinction for the great auk will be a decades long progress. Scientists must sequence the genomes of both the extinct species and its living template species and conduct comparable genomics in order to identify genes and alleles that influence phenotype (In Silico phase); successfully gene edit reproductively competent cells of the template species to possess the genetic traits of the extinct species (In Vitro phase); develop advanced reproductive technologies to generate gametes and/or embryos from the gene-edited cells (In Vivo phase); propagate de-extinct species in captivity for release to the wild (Ex situ phase); and, release captive population to the wild and begin monitoring and managing recovery (In Situ phase).
Much of the genomic resources needed to conduct In Silico phase research have already been prepared (see the genomic research section below.) While the identification of genes and alleles that influence phenotypes is still a nascent field, the major technological challenge for de-extinction is advancing from editing cells in a petri dish to generating gene-edited gametes or embryos in vivo. This proposal outlines a pathway for overcoming that challenge by developing and perfecting the advanced reproductive technologies necessary for breeding new generations de-extinct great auks. For the greak auk, an avian species, the only viable reproductive technology available is the germ-line transmission of cultured primordial germ cells, and proposed research will center on testing the technologies necessary for generating a wild bird from domestic chicken parents via the culturing and transmitting of primordial germ cells.
BIG IDEA
Developing Interspecies Germ-line Transmission for Seabirds
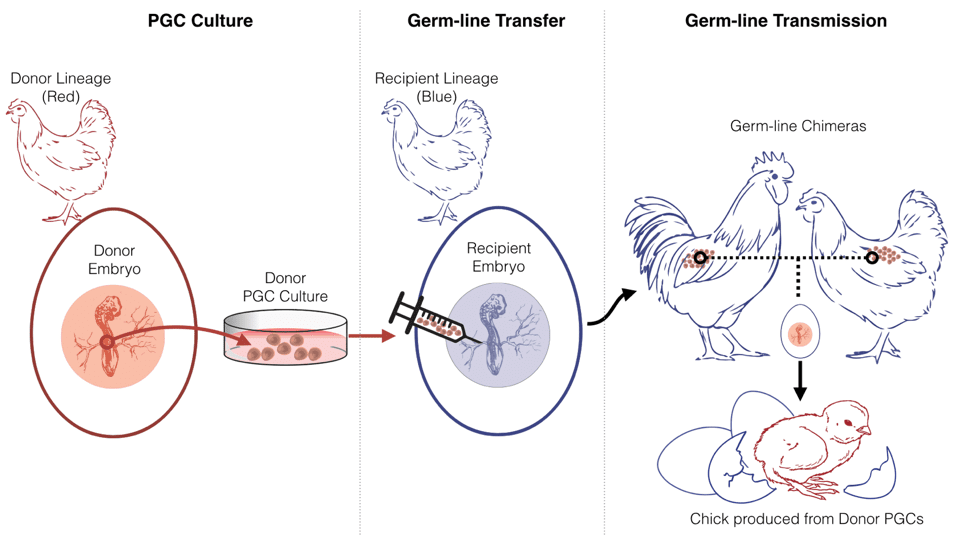
Because of avian reproductive biology, cloning or stem cell embryogenesis are not viable reproductive technologies for great auk de-extinction. Instead interspecies germ-line transmission of cultured PGCs will be used. PGCs from a donor bird (species one) are first isolated and subsequently transferred to a recipient bird (species two). This produces a germ-line chimera: a bird that generates both its own germ cells and those of the donor species. When bred, the germ-line chimera can produce offspring from the germ cells of species one.
This technology has only been developed thoroughly for the domestic chicken and limited attempts with wild species have yet to be successful. The in vivo culturing and isolation of wild bird PGCs is the first technological barrier to be overcome in order for great auk de-extinction and for genetic innovations to support avian conservation.
This project proposes to begin developing interspecies germ-line transmission for great auk de-extinction not with the razorbill but with a distantly related species more suited to captive breeding. Recent genetic analysis has proven that button-quail of the genus Turnix are in the same order as the razorbill. Therefore, button-quail present a model species to pilot germ-line transmission for its relatives, including the great auk, as it is more fecund and takes less time to sexually mature, incubate eggs, and fledge chicks than the razorbill. In addition, button-quail chicks can be raised without parental care. All of these factors reduce animal housing resources and animal care requirements for research purposes, reducing the overall costs and timelines for achieving the primary milestones.
As shown in Figure 2, the domestic chicken will be used as the surrogate parent species for germ-line transmission. Great auk de-extinction would benefit from the chicken’s long history of domestication; its adaptation to living in captivity and its high fecundity would improve the downstream establishment of a viable captive-breeding population of de-extinct great auks. Because domestic chicken breeds vary greatly from the small bantam breeds to the very large Brahmas, size is not expect to add any complications; it is expected a breed with a similar egg shell and yolk size to that of the great auk will provide a compatible reproductive surrogate.
The chicken has not yet proven to be an effective surrogate for wild species of birds, but experts hypothesize that genetically sterilized chickens could be the key to success. It is assumed that in interspecies chicken germ-line chimeras, the chicken’s native PGCs outcompete the donor PGCs of the other species. But a sterile chicken surrogate would have no germ-cells of its own, meaning that only PGCs of the donor species could proliferate and grow. Researchers at the Roslin Institute in Scotland are working to produce sterile chickens to improve germ-line transmission efficiency. This could make interspecies germ-line transmission applicable to a wide range of wild bird species.
The first milestone of this proposal is successfully culturing in vitro button-quail PGCs. The next phase of research will test the transmission of button-quail PGCs to sterile chicken recipients. If successful, this would be the first time a wild avian species was produced from domestic chicken parents. To date, wild chicks have only been produced from domestic chickens twice, and both times only from a domestic chicken father mated to a female of the wild species via artificial insemination.
Establishing interspecies germ-line transmission with the domestic chicken as surrogate could also greatly improve ex situ breeding of rare wild birds, and therefore make genetic rescue possible for endangered avian species. Where domestic chickens can produce hundreds of fertile eggs per year in captive settings, wild birds can required specialized housing to reliably breed in captivity and produce only a few fertile eggs per year. Domestic chicken germ-line chimeras could rapidly recover dwindling populations of endangered birds, and in the case of great auk de-extinction, quickly establish a viable captive breeding flock for eventual wild releases.
BUDGET
| ITEM | USD | DETAILS |
|---|---|---|
| Post Doc / Lab Tech | 300,000 | Salary at $100,000 per year for three years |
| Flock Care | 300,000 | Includes feeding, housing, cleaning, and incubation monitors; plus staff |
| Laboratory Reagents and Consumables | $75,00 | Laboratory consumables for three years, includes culturing medium and reagents. |
| Total: | $675,000 |
PARTNERS
Dr. Rosemary Waltham, Texas A&M University
Michael McGrew, Roslin Institute
Marie-Cecile Van de Lavoir, Ligand Pharmaceuticals
Tim Doran, Commonwealth Scientific and Industrial Research Organization’s Australian Animal Health Laboratory
RISKS & CHALLENGES
Reproductive techniques are difficult to develop. The reproductive biology of even closely related species can be extremely different, making collective knowledge gained in the field of reproductive biology is not always helpful when innovating techniques for new species. Developing reproductive techniques for new species is always a high risk/ high reward scientific endeavor, and this reality applies to the projects outlined in this proposal.
PGC culture conditions developed for button-quail may not foster the growth of razorbill PGCs. This would require repeating experimental procedures on germ-line transmission. The animal husbandry protocols to produce and raise gene-edited razorbills will also require a large degree of customized innovation. However, the opportunity to rapidly test the feasibility of using domestic chickens as germ-line chimeras for wild birds still has merit as a critical step in the ultimate pursuit of great auk de-extinction.
If the domestic chicken cannot adequately transmit razorbill PGCs, there are alternatives for great auk de-extinction. Germ-line transmission of gene-edited razorbill PGCs within razorbill germ-line chimeras should work.
A potential problem with using razorbill germ-line chimeras to breed great auks is that the egg shell will be too small to hatch offspring gene edited for great auk body size. Breeding a heterozygote intermediate may provide a solution, as there are similar examples of hybridization between birds of differing size. There is another solution; early stage great auk embryos can be excised from razorbill yolks and grafted onto larger yolks and incubated in the eggs of a compatible species, such as the domestic chicken breed originally intended as an interspecies chimera. Given that interspecies embryo transplants are routinely performed for ex ovo embryological studies, there is reason to expect that this technique can be used to viably grow de-extinct great auk embryos.
TIMELINE
Year 1
- Set up an experimental breeding flock of button-quail as a source of fertile eggs for experimentation.
- Analyze PGC migration in relation to embryonic growth stages to isolate PGCs at multiple time points for culture experiments.
- Test multiple culture conditions.
- Test any viable cell cultures for PGC specific gene markers.
MILESTONES:
a) Continual egg production from breeding flock via controlled lighting.
b) Embryonic growth chart of the button-quail
c) Validation of germ cell gene markers in several cell cultures.
Year 2
- Transfect PGC cultures with green Ffuorescent protein to aid as a visual marker for in vivo observations.
- Inject fluorescent PGCs into domestic chicken embryos to validate proper in vivo migration.
- Inject fluorescent PGCs into button-quail embryos to test reproductive germ- line transmission. Injected embryos will be hatched, raised to sexual maturity, and bred to produce fluorescent chicks (these would be the first genetically engineered wild birds ever hatched)
MILESTONES:
a) Validation of PGC cultures by in vivo activity testing.
b) Genetically engineered PGCs (fluorescent PGCs)
c) Fluorescent genetically engineered button-quail hatchlings.
Year 3
- Test germ-line transmission in normal domestic chickens. If process doesn’t work, repeat test with sterilized chickens.
MILESTONES:
a) Fluorescent genetically engineered button-quail bred from domestic chicken
surrogate parents.